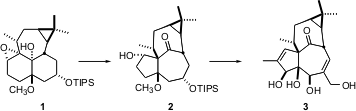
The total synthesis of the tetracyclic Euphorbia tetraol ingenol 3 reported by Keiji Tanino of Hokkaido University (J. Am. Chem. Soc. 2003, 125, 1498.DOI: 10.1021/ja029226n)illustrates the power of diastereoselective carbocationic rearrangements, as exemplified by the conversion of 1 to 2. 1-Bromo-3,4-difluoro-2-methoxybenzene In stock
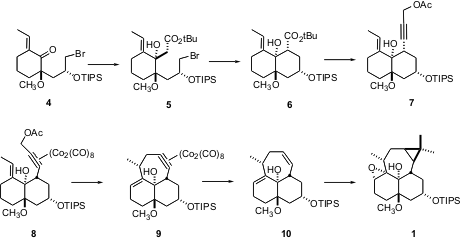
The construction of the tricyclic epoxide depended on several highly diastereoselective transformations. 298-06-6 site The addition of lithiot-butyl acetate to ketone 4 proceeded to give 5 as a single diastereomer, even though the ketone is flanked by a quaternary center. The authors speculate that lithium chelation with the methyl ether directed addition. Even more spectacular was the cyclization of the propargylic acetate 7 to 10. The Co complex activated the acetate for ionization, while at the same time establishing the proper geometric relationship for bond formation. Dissolving metal reduction of the Co complex then gave the alkene.
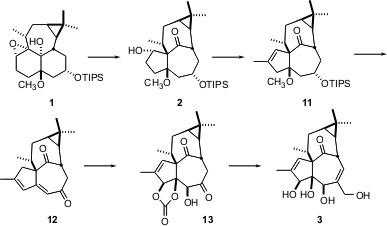
The elegant pinacol rearrangement of 1 to2, mediated by (ArO)2AlCH3, exposed a ketone that might usually need to be protected. In this case, however, the ketone is so buried in the inside-outside ingenol skeleton that it is unreactive. After several further manipulations, a spectacular osmylation of the diene 12 led to ingenol 3, in an overall 45-step sequence.
The ingenol 3 prepared by this route was racemic. It is interesting to speculate how one might efficiently prepare 4 or its precursors in enantiomerically-pure form.